How to Find Moles of Water Formed in a Reaction
If 602 x 10 23 molecules 1 mole of pentane are burned 8602 x 10 23 molecules 8 moles of oxygen are needed. Average initial temp 29565K.

Question Video Calculating The Mass Of Water Produced Given The Masses Of Oxygen And Hydrogen Nagwa
Calculate the heat of reaction per mole of water formed in the acid-base reaction.

. 120g Al 1 mol Al 2698g Al 0044 48 mol Al. Molarity moles of solute litres of solution. The reaction would form 5602 x 10 23 molecules 5 moles of carbon dioxide and 6602 x 10 23 molecules 6 moles of water.
The balanced chemical equation is HCl aq NaOH aq -------- NaCl aq H2O l As per the stoichiometric equation 1 mole HCl 1 mole NaOH 1 mole H2O. H Cl N aOH H 2O N aCl. Find the number of mole.
Multiply the ratio obtained in Step 2. From the stoichiometry of this equation one mole of Na deposited requires the passage of one mole of electrons in the electrolysis. View the full answer.
Calculate the mass in grams of each reactant. Now you have to find out the molar mass M of water H 2O. To calculate for the moles of water produced in the reaction we first need to find the limiting reagent in the reaction.
A Moles of Mg OH₂. Divide Grams by Grams per Mole. From the reaction equation you can now determine the amount of substance of water that will be produced.
This appears to be far too much water as 1 mole of water weighs approx 18g and 17 moles of water would weigh about 310g much more than the reaction mixture. Determine the mole fraction of NaCl in a solution in which 010 moles of the salt is dissolved in 100 grams of water. Thus 1023 mole of water is consumed in this reaction and 167-1023 0647 moles of water remains reacted.
I assume you mean this reaction2H2 O2 - 2H2O22 moles O2 2 moles H2O1 mole O2 44 moles water made. H 101 gmol O. 1 1 1 1 mole.
NceH2Oapprox 28mathrmmol You can convert that easily into a mass now. View the full answer. Assuming the value is -562 kJ per mole of water formed and your value is - 3260 kJ you can find the number of moles of water by 5623260 17239 moles of water.
Calculate the molar ratios. Express your answer with the appropriate units. The first and last numbers are two times Avogadros number while the second number is Avogadros number.
B Moles of H₂O. The moles of NaCl is provided but you still need the number of moles of water H 2 O. Comparative Analysis and Facts.
The molar ratio is 2 mol H₂O 1 mol Mg OH₂. To use all the Na 2 O 3 1023 moles of water will be required. How to Calculate Moles in a Reaction.
Final temp 3088K Homework Equations ΔH rxn q rxn -mcΔT The Attempt at a Solution. HCl Δ N H q N n x Δ N H -297064 J 005mol Δ N H -594kJmol HNO 3 Δ N H q N n x Δ N H -255224 J 005mol Δ N H -510kJmol. Moles of Cu deposited 100 6355 1574 x 10 2 mol so moles of electrons passed 2 x 1574 x 10 2 3148 x 10 2 mol.
240g I₂ 1 mol I2 2538g I₂ 0009 456 mol I2. Assume that the total mass of the solution is 150g. Convert all masses into moles.
Calculate the number of moles of water formed when you use all available oxygen Calculate the number of moles of water formed when you use all available oxygen 74 rm mol. Peptide Bond vs Disulfide Bond. Determine the heat of neutralization per mole of water.
Molar ratio Moles and Chemical Reactions Chapter 4. Correct 158 rm mol of hydrogen can form 158 rm mol of water. The moles of electrons used 2 x moles of Cu deposited.
Darmaidayxx and 3 more users found this answer helpful. The reaction will form of water. Determine the atomic weight of each element using the periodic table.
Compare the heats of neutralization per mole of water for the two strong acids. If 1 mole of HCl or NaOH gives you 1 mole of H 2O then the number of moles in H 2O is. 501a How many Moles of water are produced 111.
To know more please go through. Start by calculating the number of moles in one gram of water using periodic table data for hydrogen and oxygen. Moles and Chemical Reactions Chapter 4 mol NH 3 10 mol H 2 x 2 mol NH 3 3 mole H 2 067 mol NH 3 Moles and Chemical Reactions Chapter 4 If you have 10 mole of H 2 how many moles of N 2 will be required to completely react all of the H 2.
Looking at the equation. But these numbers are related to the number of things in a mole. The number of moles of water produced is equal to the number of moles of hydrogen gas consumed.
First of all before you can use this equation you need to know how many moles of solute are there in the solution. Since the density of water is 1 Kg L a liter of water has the mass of 1 Kg 1000 g. For finding out this you have to multiply the mass of solute by its molar mass conversion factor.
To calculate the molar ratios you put the moles of one reactant over the moles of the other reactant. We will also explore limiting reagents and percent yield to address practical aspects of chemical reactions because there may be leftover reactants andor incomplete formation of products. Find Mass in Grams.
Since all compounds in this reaction have 1 mole it will not affect the number of moles n. 501 Stoichiometry of Chemical Reactions. MceH2Oapprox 50mathrmmol There are two notable changes you will observe.
1 mol H 2 Find. These coefficients are also in the ratio of 212. Calculate Grams per Mole.
Find out the moles of HCl and NaOH as b. How many moles of water are made form the reaction of 22 moles of oxygen gas. 12044 1023 H2 6022 1023 O2 12044 1023 H2O.
Mol N 2 Conversion factor. 501b How many Moles of CO2 are produced 147. The number of moles of substance is calculated by dividing the mass in grams by the mass of one mole which corresponds to the molecular weight expressed in grams.
N2 3 H 2 2 NH 3 Given. Since you are combusting a gas obviously no bubbles can appear. Mole to Mole 819.
You dont tell us what the reaction is but we can solve the problem so long as we balance the OH. The number of moles of water is 1000g. 75mL of 20M NaOH added to 75mL of 20M HCl in a styrofoam cup.
Enthalpy of neutralization of weak acid and strong base reaction 2 -q neutralization q cal qcal CcalxAT AT3322C- 200C 1322 C -q neutralization 4184 JC X 1322C. When a mole of pentane undergoes complete combustion How many moles of water are formed.

How To Find Moles Of Product From Moles Of Reactant Using A Chemical Equation Chemistry Study Com
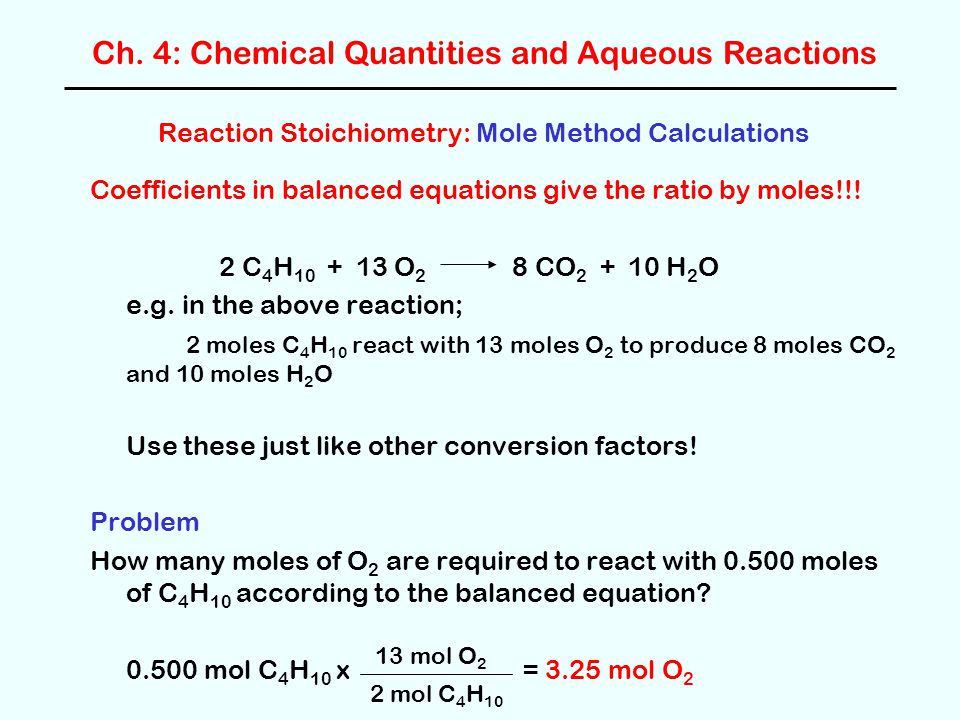
Reaction Stoichiometry Mole Method Calculations Ppt Download

Aleks Using A Chemical Equation To Find Moles Of Product From Moles Of Reactant Youtube
No comments for "How to Find Moles of Water Formed in a Reaction"
Post a Comment