Which One of These Four Atoms Has the Most Neutrons
Thats why the neutrons in the diagram above are labeled n0. A vague meaning at first.
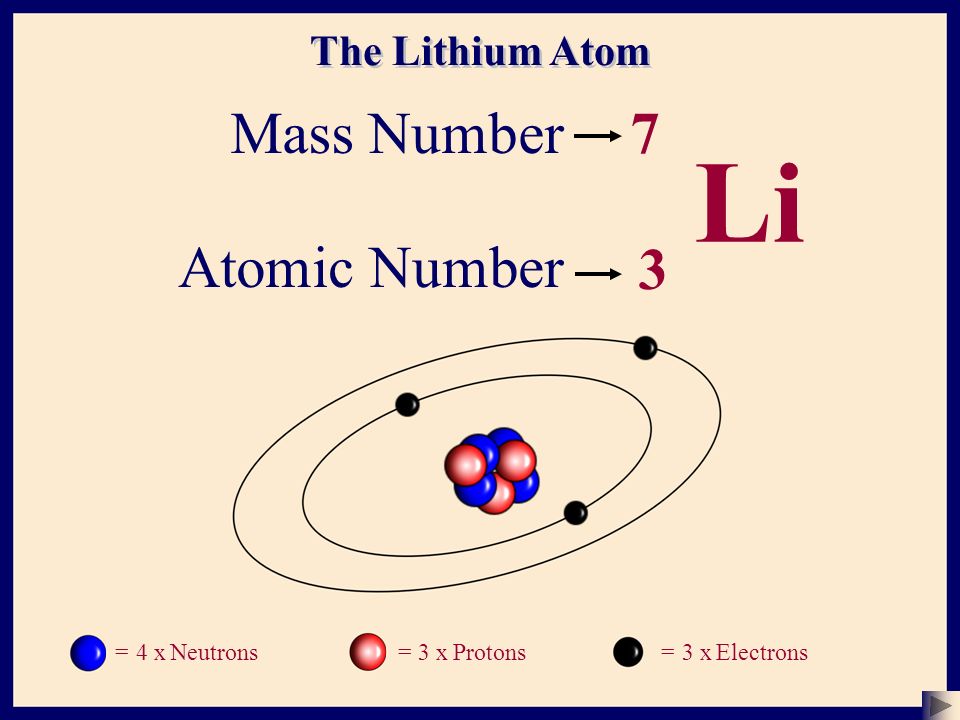
The Atom Basic Structure 1 Ppt Download
The nuclide concept referring to individual nuclear species emphasizes nuclear properties over chemical properties whereas the isotope concept grouping all atoms of each element emphasizes.
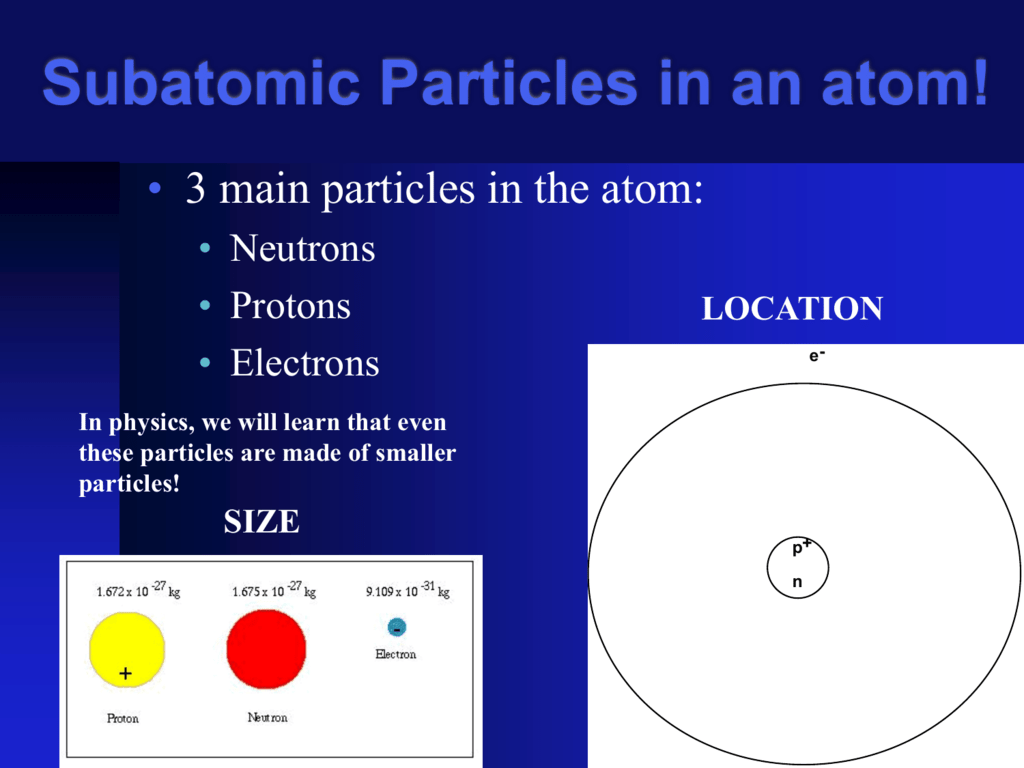
. The vogue for the word used until the late 18th century only in Latin. Atoms of all elementsexcept for most atoms of hydrogenhave neutrons in their nucleus. In tritium the concentration is one atom per 1018 atoms of protium.
Unlike protons and electrons which are electrically charged neutrons have no chargethey are electrically neutral. The most prominent form of hydrogen is protium 00156 of hydrogen is present on the earths surface as deuterium. Out of these three isotopes of hydrogen Out of these three isotopes of hydrogen the only tritium is radioactive in nature which emits low energy b particles.
Molecule 1794 extremely minute particle from French molécule 1678 from New Latin molecula diminutive of Latin moles mass barrier. According to Merriam-Webster and the Online Etymology Dictionary the word molecule derives from the Latin moles or small unit of mass. The zero stands for zero charge.
A nuclide is a species of an atom with a specific number of protons and neutrons in the nucleus for example carbon-13 with 6 protons and 7 neutrons.
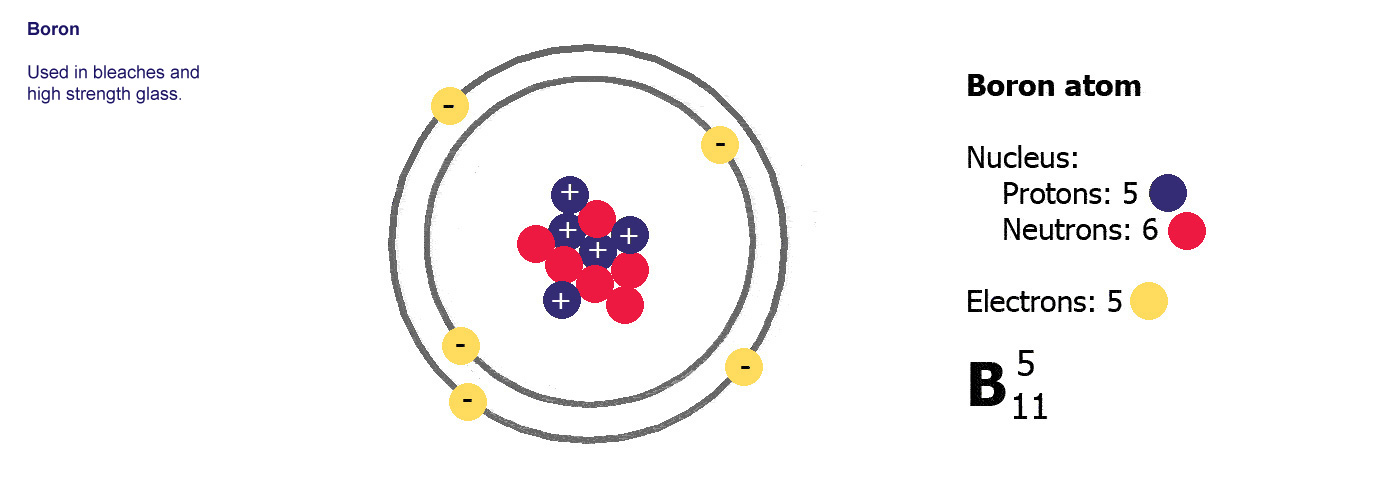
Atoms The Building Blocks Of Matter
No comments for "Which One of These Four Atoms Has the Most Neutrons"
Post a Comment